Next
Gay-Lussac studied chemical reactions in gaseous phase. One of his observations was related to the formation of water...
Let us assume 1 volume of any gas to have the same number of atoms at identical temperature and pressure. Remember the formula of water was not known back then. Whether you assume it to be HO or H2O, it would require the splitting of oxygen atom to support the formation of 2 volumes of water.
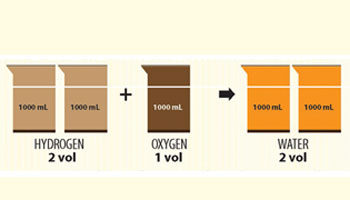
Such experimental observations by Gay-Lussac contradicted the concept of atom proposed by Dalton.
This contradiction was brilliantly resolved by Avogadro in 1811, who suggested that the smallest particle of the gas could sometimes be a combination of two or more atoms. You now know that Avogadro was talking about the molecule. His famous hypothesis namely ‘equal volumes of all gases under the same conditions of temperature and pressure contain equal number of molecules’ also solved the problem of counting atoms.
Cannizzaro fruitfully used Avogadro’s hypothesis for the determination of molecular and atomic weights. He determined the volume occupied by 1 mole of a gas at 298K, 1 atm. pressure to be 22.4 litres. Using this constant, density and elemental analysis of gaseous compounds, he could determine the weights of the elements.

Another scientist who contributed significantly and independently towards determination of atomic weights is Berzelius.

He prepared and analysed more than 2000 compounds, mostly oxides. He changed the reference from hydrogen to oxygen and considered the formula of water as H2O. (today 12C isotope is the reference for atomic weights).
Next
Previous: Dalton's Atomic Theory